THE I-MEQ™ STRATEGY
What is I-MEQ™?
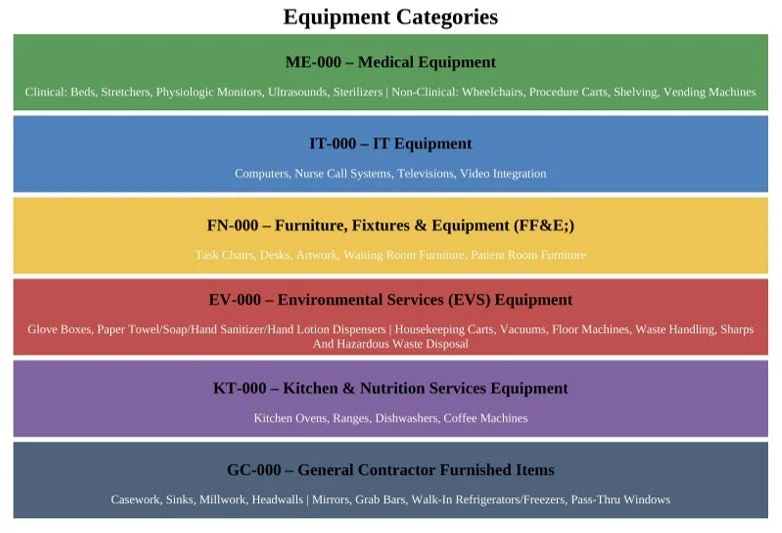
Most medical equipment planning (MEQ) projects begin by focusing on Medical Equipment and Environmental Services (EVS), the traditional anchors of equipment planning. However, limiting planning to just these two categories often leaves out critical elements—such as IT Equipment and FF&E—which can lead to overlaps, blurred responsibilities, and costly late fixes. I-MEQ™ bridges these gaps with a complete, integrated approach that extends beyond MEQ and EVS to include IT Equipment, FF&E, Construction, and Kitchen/Dietary. This ensures every item in the room is accounted for, creating a smoother, more efficient project from start to finish.
I-MEQ™ EVOLUTION
In 2015, the adoption of Integrated Equipment Planning practices was guided by the Owner’s discretion.
Adopting a Medical Equipment Responsibility Matrix (MER) is essential to clearly define functional accountabilities across the project team.
In 2019, Integrated Equipment Planning evolved with the collaboration of the Owner, the Architect, and Supply Chain Management.
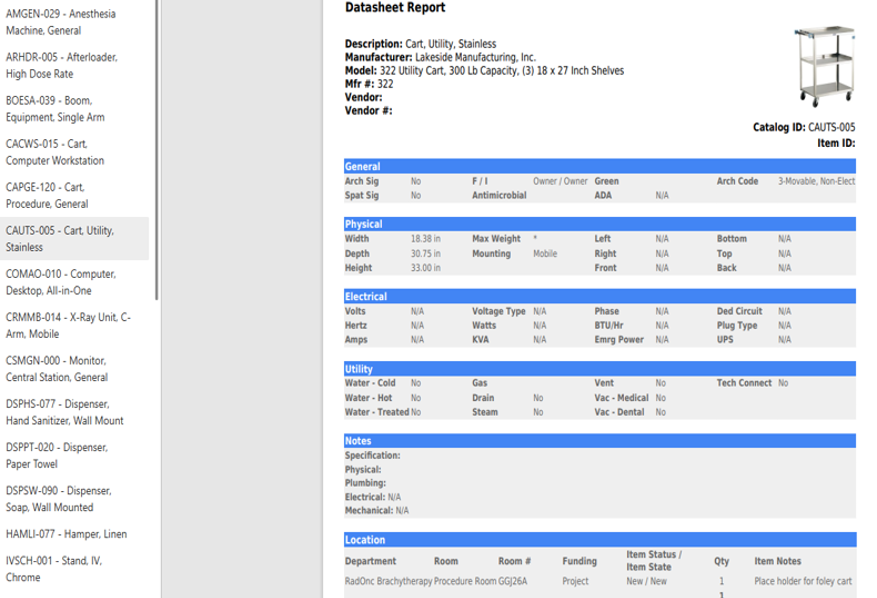
User-friendly equipment databases were implemented to provide the project team with easy access to information and detailed specifications.
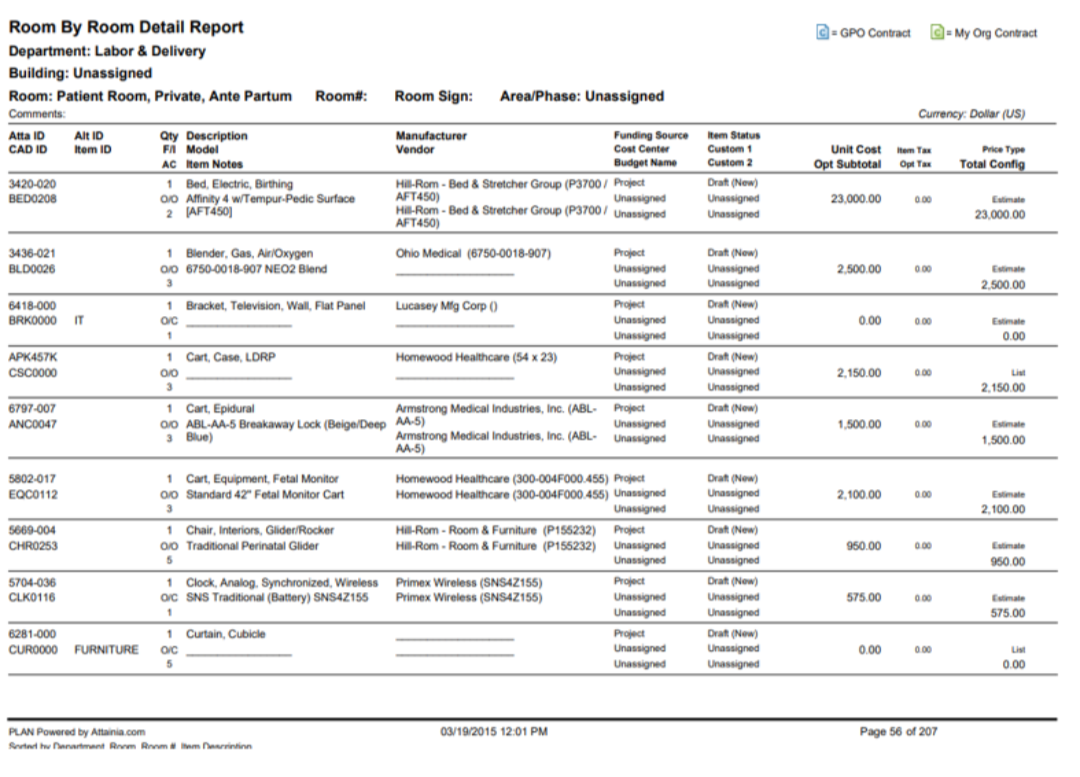
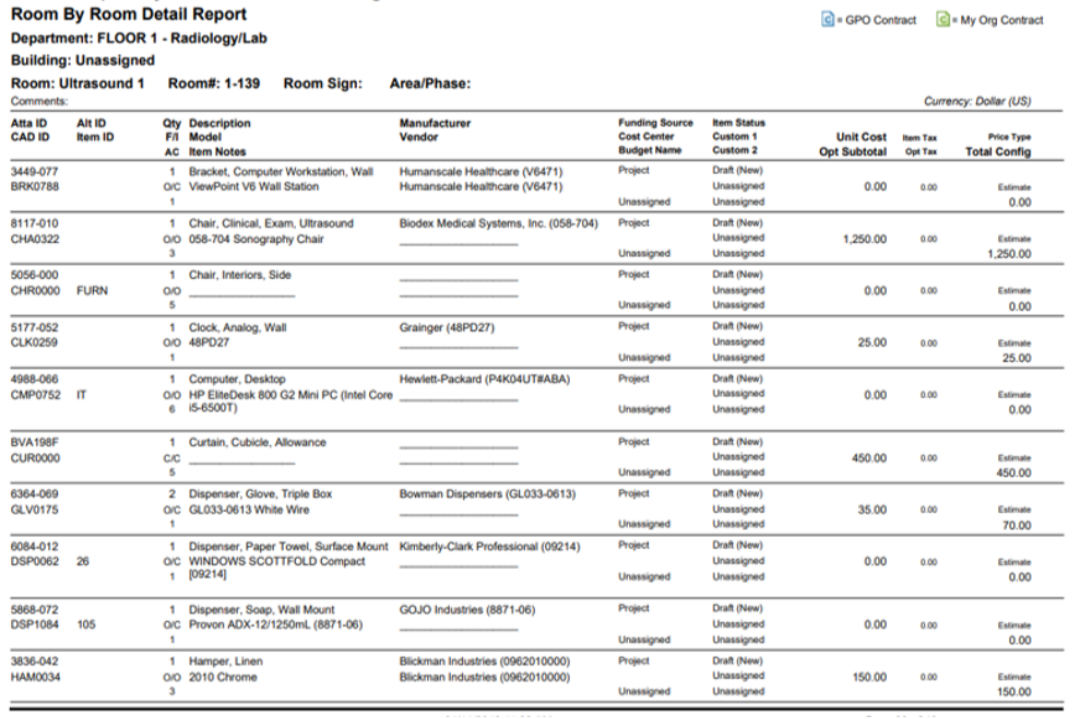
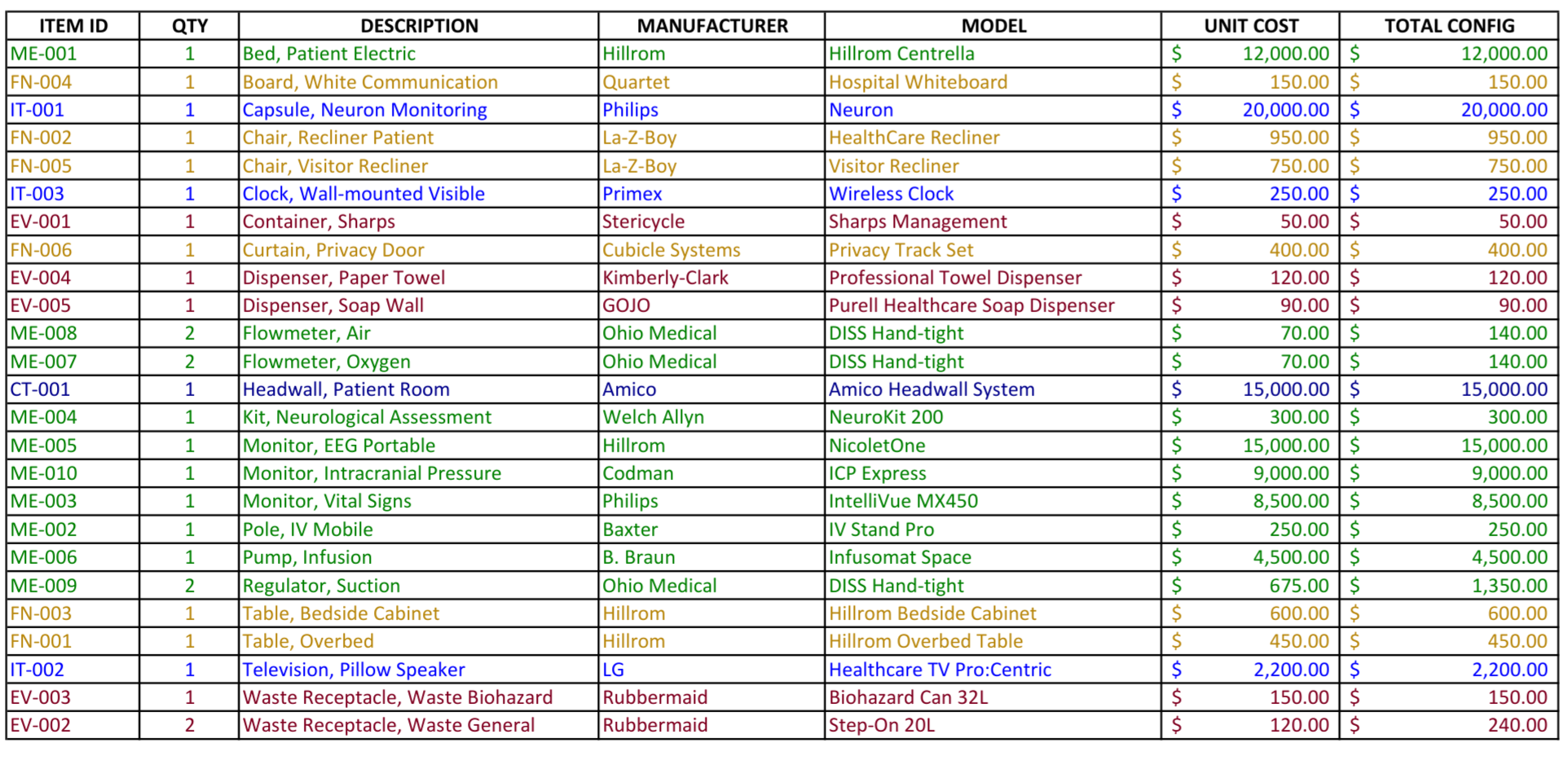
This Neurology Patient Room Equipment List represents the mature, data-driven realization of the I-MEQ™ methodology, where Medical Equipment (MEQ), IT Equipment, FF&E, EVS, Construction, and Kitchen/Dietary categories are no longer treated as separate silos, but as a coordinated and accountable ecosystem.

Driving Consensus
By consolidating all equipment categories into the I-MEQ™ Database, project teams gain real-time clarity, eliminate duplication, and ensure consistent accountability across all stakeholders.
When gaps exist, mistakes follow—duplicate furniture orders, monitoring lost between IT and CE, headwalls left in limbo, nurse call and ceiling lifts slipping through the cracks. These errors drain money, waste time, and erode credibility. With I-MEQ™, they vanish—because every discipline is aligned, every stakeholder works from the same truth, and every question is answered together, early. Consensus is no longer elusive—it emerges naturally from the process.
I-MEQ™
PROCESS
Select the (+) button beside each section to explore details about the I-MEQ™ processes.
-
The groundwork for a successful project begins here. Early planning ensures that all disciplines—Medical Equipment, IT Equipment, FF&E, EVS, Construction, and Kitchen/Dietary—are identified and considered from the outset.
-
During this phase, each discipline’s requirements are integrated into a single, coordinated plan. This early collaboration creates a unified database—a reliable single source of truth—that sets clear expectations, drives accountability, and keeps everyone on the same page.
-
With all elements already captured and coordinated, the project moves seamlessly into Design Development. The team can proceed with confidence, knowing every detail has been confirmed and there are no costly surprises down the road.
The I-MEQ™ Solution
This is what I-MEQ™ delivers: one seamless wall, strong and complete. From Concept Development through activation, every discipline—Medical Equipment, IT Equipment, FF&E, EVS Items, Construction, and Kitchen/Dining—is united into a single, coordinated plan. The I-MEQ™ Database, as shown above, brings all these elements together to provide one true source of truth for your healthcare design project.
With I-MEQ™, you get reliable results, accountable processes, and total readiness on day one. No gaps, no overlaps, no costly surprises—just a fully integrated solution that keeps your team aligned and your project on track.
I-MEQ™ practices a strict “No Gap” strategy. Every item in the room is categorized as predetermined in the MER.
I-MEQ™: Reliable. Accountable. Zero Gaps.